Chapter 4.23.1
4.23.1 - Optimizing legume productivity using molybdenum fertilizer
Dylan P. Harding, University of Guelph, Canada
Suggested citation for this chapter.
Harding,DP. (2022) Optimizing legume productivity using molybdenum fertilizer. In Farmpedia, The Encyclopedia for Small Scale Farmers. Editor, M.N. Raizada, University of Guelph, Canada. http://www.farmpedia.org
Introduction
Rather than having to purchase synthetic nitrogen fertilizer, farmers can take advantage of certain types of naturally occurring soil bacteria (“nitrogen fixing bacteria” or “rhizobial/Rhizobium” bacteria) that can infect specialized root organs, called nodules, in legume plants (e.g. beans, lentils) and green manures (e.g. alfalfa, clover). Once rhizobial bacteria have successfully “infected” a plant, they can convert naturally occurring atmospheric nitrogen gas (N2) to plant-available nitrogen in the form of ammonia. The nitrogen fixation reaction uses an enzyme called Nitrogenase which preferentially employs the mineral molybdenum (Mo), and thus the presence of this element in the soil is essential for successful fixation of atmospheric nitrogen (Bambara & Ndakidemi, 2010). If lacking in the soil, molybdenum as well as improved strains of nitrogen fixing bacteria can be coated onto legume seeds as a powder or by soaking seeds in a molybdenum solution prior to planting. As molybdenum is required in only trace amounts, this is a generally cost-effective intervention.
Effect of Molybdenum on Crops and Application Methods
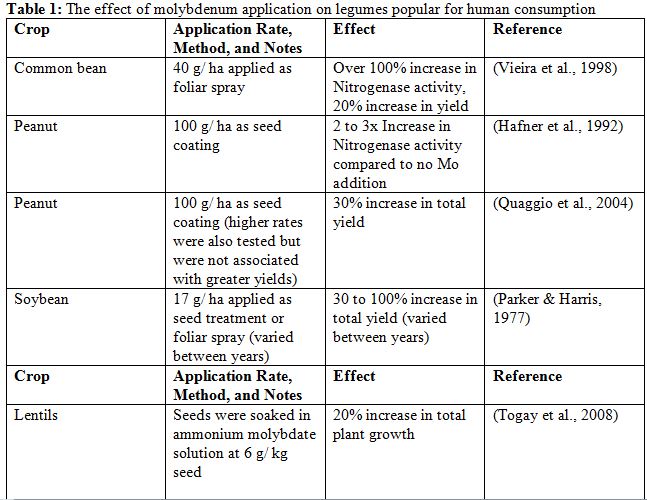
Where deficient, the application of molybdenum has been shown to increase the yield and nitrogen fixation ability of legumes in many studies (Bailey & Laidlaw, 1999; Hafner, Ndunguru, Bationo, & Marschner, 1992; Jongruaysup, Dell, Bell, Ohara, & Bradley, 1997; Lopez, Alvear, Gianfreda, & Mora, 2007; Quaggio, Gallo, Owino-Gerroh, Abreu, & Cantarella, 2004; Rosolem & Caires, 1998; Togay, Togay, & Dogan, 2008; Vieira, Cardoso, Vieira, & Cassini, 1998; Vistoso, Alfaro, & Mora, 2012). The effects of various molybdenum application rates and methods on legumes popular for human consumption are summarized in Table 1
It should be noted that the molybdenum seed treatment method employed in several of the above studies involved soaking the seeds before transplanting (Johansen, 2005; Togay et al., 2008). Soaking seeds alone has been shown to improve germination and growth for some species (Aune & Ousman, 2011; Ousman & Aune, 2011). As such, the soaking method of applying molybdenum to the seed may have caused a falsely positive effect on the observations drawn regarding the effect of Mo in these studies.
It has been observed that neither broadcast nor seed-application of molybdenum is necessarily a more effective method of Mo application, and that the ideal method of Mo application for achieving maximum nodulation will vary between fields (Johansen, 2005). With this in mind, simple field trials should be conducted at a given site to determine the ideal method of Mo application where practical.
Identifying Molybdenum Deficiency
Conventional soil testing methods are not effective or often available for molybdenum (McKenzie, 1966). As molybdenum deficiency will interfere with nitrogen fixation in legumes, symptoms of nitrogen deficiency in these crops (yellowing and tip drying of older leaves) can be a simple indicator of molybdenum deficiency, though other factors such as the absence of nitrogen fixing bacteria or acidic soil conditions (see below) can cause the same symptoms. Legume nitrogen fixation can be more accurately estimated by gently digging up the root system and counting the number of root nodules. Nodules have the appearance of small beads on the roots. Nodules that are actively fixing nitrogen appear red (inside and/or out) and this colour in particular, along with nodule number and size, indicates the total level of nitrogen fixation per plant. To gauge whether there may be a molybdenum deficiency, nodule observations from fields with low-yielding legumes should be compared with fields with high-yielding crops of the same species.
Molybdenum may be present in the soil but not available to crops when the soil is acidic thus adding more molybdenum may not be beneficial under these conditions (Franco & Munns, 1981). For example, common beans (Phaseolus vulgaris) have been shown to not respond to Mo addition when the pH is below 5.2 (Franco & Munns, 1981). Soil acidity can be estimated using inexpensive litmus paper.
If the cause of low yielding legumes remains unclear, a small test plot of legumes should be planted, in which half will receive an addition of molybdenum and half will not. Ideally, the farmer and whoever is measuring yield should not know which sub-plot received the treatment (“blind study”) so that the treatment of the entire plot is as consistent as possible once established.
Improving Molybdenum Availability Using Liming
Applying lime to the soil is common practice to overcome acidic soils and has also been shown to improve plant molybdenum uptake by several studies (Franco & Munns, 1981; Lopez et al., 2007; Mandal, Pal, & Mandal, 1998; Quaggio et al., 2004). In a field trial carried out with peanuts on soil with an initial pH of 4.2, liming at a rate of 2 t/ha was found to be equally effective as molybdenum seed coating without liming (Quaggio et al., 2004). This suggests that if molybdenum is already present in the soil but unavailable because of acidic conditions, applying molybdenum with the seed may be unnecessary if the soil can be treated with lime. Similarly, a field trial with Egyptian clover on an alkaline soil (pH 8.15) and an initial soil molybdenum concentration of 0.18 mg/ kg found no statistically significant yield benefit from a molybdenum leaf spray, suggesting that molybdenum availability is less problematic when soil is not acidic (El-Bably, 2002). It should be noted however that urea (a nitrogen containing fertilizer) was also applied to the experimental fields in this study, obscuring the effect of molybdenum on nitrogen fixation (El-Bably, 2002).
Drawbacks and Limitations
Molybdenum has been shown to enhance the beneficial effects of rhizobial inoculation in comparison to inoculation alone (Bambara & Ndakidemi, 2010; Johansen, 2005), however at high application rates (e. g. more than 30 micrograms per litre of seed priming water), molybdenum was shown to decrease the symbiotic interaction between rhizobia and pea (Pisum-sativum) (Chahal & Chahal, 1991). High doses of molybdenum applied directly to the seed may discourage symbiotic interaction between rhizobium and other bacteria species as well, so it is important to be careful not to use too much molybdenum when adding it directly to a seed preparation.
Additional Notes on Interactions between Inoculation and Mo Seed Coating
Although neither seed coating nor soil application of Mo was consistently more effective in improving nodulation of chickpea than the other, both application methods improved nodulation in comparison to inoculation alone (Johansen, 2005). This suggests that seed coating with molybdenum does not necessarily interfere with successful rhizobial inoculation. It should be noted that acidity was not a limiting factor in this study (Johansen, 2005), and that acidic conditions may favour Mo seed coating because Mo will be closest to the seed with this application method.
It was found in another study that applying Molybdenum with seed inoculants increased yield benefits with increasing Mo rates. Yield from inoculated seeds were higher than yields from uninoculated seeds, indicating a positive effect from combination of Mo seed coating and inoculation in this study (Bambara & Ndakidemi, 2010).
It should be noted also that as with seed-placed fertilizer, seed-coating will decrease moisture availability to the seed. For this reason seed-coating is not recommended in situations where available moisture is likely to limit germination rates.
As a cautionary note, when acidity related unavailability is not as issue, there is potential for molybdenum toxicity to occur to the plant (Brenchley, 1948) so molybdenum additions should be carefully measured as a seed preparation is made.
Practical tips
Molybdenum can be coated onto seeds at the same time as rhizobial inoculatants. A method for the preparation of inoculant seed coatings is outlined in the May 2010 N2 Africa Workshop report (Saidou Koala, Baijukya, Qureish Noordin, Abdullahi Bala, & Ngokho, 2010). This guide does not include molybdenum in its seed coating preparation method however powdered molybdenum fertilizer such as ammonium molybdate could be substituted for a portion of the mineral mix. An adhesive should also be employed to ensure lasting contact between the seed and its coating (Saidou Koala et al., 2010).This adhesive can be prepared using locally available materials such as gum Arabic, granular sugar or sugarcane molasses. Please see Table 1 (page 16) of this guide (link provided below) for the correct ratios of adhesive, water and inoculant/mineral mix. The preparation of the seed coating will also require plastic bags without holes or a bucket with a lid for larger volumes of seed.
Molybdenum can also be broadcast or applied as a foliar spray. Foliar spray application has the advantage of avoiding the pH-dependant availability issues associated with molybdenum however the potential for over-application and resultant toxicity is higher with this method. It must be kept in mind again however that this risk is present for all methods of molybdenum application. Seed preparations through coating or soaking with molybdenum are likely the least labour intensive methods of Mo fertilization for most situations, and have the additional advantage of encouraging even application and proximity of Mo to the seed. The most effective method for fertilizing with Molybdenum will vary between fields (Johansen, 2005). As such, determining the ideal molybdenum application method is best achieved through small scale field testing, although this will require waiting through the test season before molybdenum application can be applied to production fields if determined necessary.
Molybdenum fertilizer can be purchased in several forms. Commercially prepared micronutrient mixes containing molybdenum are available however these are often unavailable to poor farmers. Isolated forms of molybdenum fertilizer also exist. These sources will contain either a very simple mineral form of molybdenum such as ammonium-molybdate or sodium-molybdate, or as molybdenum prepared into a “chelated” compound. Chelated compounds are those in which the molybdenum mineral has been incorporated into a more complex carbon molecule in order to enhance plant uptake and availability. Although this preparation may increase molybdenum availability, simple mineral forms of molybdenum have also been shown to be effective.
Additional Resources
This workshop report from the N2 Africa organization suggests a seed coating method into which Mo fertilizer could be incorporated. Related YouTube videos are also available through this link: http://www.n2africa.org/N2media.
Listing of Fertilizer Producers in India: http://agriculture.indiabizclub.com/manufacturer/bio_fertilizers.
References
1.Aune, J. B. and A. Ousman (2011). "EFFECT OF SEED PRIMING AND MICRO-DOSING OF FERTILIZER ON SORGHUM AND PEARL MILLET IN WESTERN SUDAN." Experimental Agriculture 47(3): 419-430.
2.Bailey, J. S. and A. S. Laidlaw (1999). "The interactive effects of phosphorus, potassium, lime and molybdenum on the growth and morphology of white clover (Trifolium repens L.) at establishment." Grass and Forage Science 54(1): 69-76.
3.Bambara, S. and P. A. Ndakidemi (2010). "Phaseolus vulgaris response to Rhizobium inoculation, lime and molybdenum in selected low pH soil in Western Cape, South Africa." African Journal of Agricultural Research 5(14): 1804-1811.
4.Chahal, V. P. S. and P. P. K. Chahal (1991). INTERACTION STUDIES BETWEEN RHIZOBIUM-LEGUMINOSARUM AND MELOIDOGYNE-INCOGNITA ON PEA (PISUM-SATIVUM L) GROWN UNDER DIFFERENT CONCENTRATIONS OF MOLYBDENUM. Dordrecht, Kluwer Academic Publ.
5.El-Bably, A. Z. (2002). "Effect of irrigation and nutrition of copper and molybdenum on Egyptian clover (Trifolium alexandrnium L.)." Agronomy Journal 94(5): 1066-1070.
6.Franco, A. A. and D. N. Munns (1981). "RESPONSE OF PHASEOLUS-VULGARIS L TO MOLYBDENUM UNDER ACID CONDITIONS." Soil Science Society of America Journal 45(6): 1144-1148.
7.Hafner, H., et al. (1992). "EFFECT OF NITROGEN, PHOSPHORUS AND MOLYBDENUM APPLICATION ON GROWTH AND SYMBIOTIC N2-FIXATION OF GROUNDNUT IN AN ACID SANDY SOIL IN NIGER." Fertilizer Research 31(1): 69-77.
8.Johansen, C., Musa, A. M., Kumar Rao, J.V.D.K., Harris, D., Ali, M.Y., & Lauren, J.G. (2005). Molybdenum response of chickpea in the High Barind Tract (HBT) of Bangladesh and in Eastern India. In Andersen, P., Tuladhar, J. K., Karki, K. B., Maskey, S. L. (Eds.), Micronutrients in South and South East Asia.(pp 205-220). Kathmandu, Nepal: International Centre for Integrated Mountain Development.
9.Jongruaysup, S., et al. (1997). "Effect of molybdenum and inorganic nitrogen on molybdenum redistribution in black gram (Vigna mungo L Hepper) with particular reference to seed fill." Annals of Botany 79(1): 67-74.
10.Lopez, R. S., et al. (2007). "Molybdenum availability in andisols and its effect on biological parameters of soil and red clover (Trifolium Pratense L.)." Soil Science 172(11): 913-924.
11.Mandal, B., et al. (1998). "Effect of molybdenum, phosphorus, and lime application to acid soils on dry matter yield and molybdenum nutrition of lentil." Journal of Plant Nutrition 21(1): 139-147.
12.McKenzie, R. M. (1966). "The relation of laboratory analyses for copper, zinc, and molybdenum in some Victorian soils to the results of field trials." Australian Journal of Experimental Agriculture and Animal Husbandry 6(May 1966): 170-174.
13.Ousman, A. and J. B. Aune (2011). "EFFECT OF SEED PRIMING AND MICRO-DOSING OF FERTILIZER ON GROUNDNUT, SESAME AND COWPEA IN WESTERN SUDAN." Experimental Agriculture 47(3): 431-443.
14.Parker, M. B. and H. B. Harris (1977). "YIELD AND LEAF NITROGEN OF NODULATING AND NON-NODULATING SOYBEANS AS AFFECTED BY NITROGEN AND MOLYBDENUM." Agronomy Journal 69(4): 551-554.
15.Quaggio, J. A., et al. (2004). "Peanut response to lime and molybdenum application in low pH soils." Revista Brasileira De Ciencia Do Solo 28(4): 659-664.
16.Rhodes, E. R. and M. Kpaka (1982). "EFFECTS OF NITROGEN, MOLYBDENUM AND CULTIVAR ON COWPEA GROWTH AND YIELD ON AN OXISOL." Communications in Soil Science and Plant Analysis 13(4): 279-283.
17.Rosolem, C. A. and E. F. Caires (1998). "Yield and nitrogen uptake of peanuts as affected by lime, cobalt, and molybdenum." Journal of Plant Nutrition 21(5): 827-835.
18.Togay, Y., et al. (2008). "Research on the effect of phosphorus and molybdenum applications on the yield and yield parameters in lentil (Lens culinaris Medic.)." African Journal of Biotechnology 7(9): 1256-1260.
19.Vieira, R. F., et al. (1998). "Foliar application of molybdenum in common beans. I. Nitrogenase and reductase activities in a soil of high fertility." Journal of Plant Nutrition 21(1): 169-180.
20.Vistoso, E. M., et al. (2012). "Role of Molybdenum on Yield, Quality, and Photosynthetic Efficiency of White Clover as a Result of the Interaction with Liming and Different Phosphorus Rates in Andisols." Communications in Soil Science and Plant Analysis 43(18): 2342-2357.