Chapters 4.16.1
4.16.1 - Synthetic fertilizers: primer and raising yields
Dylan P. Harding, University of Guelph, Canada
Source:https://en.wikipedia.org/wiki/File:Manure_spreading_Hlokozi_2007_11_29.jpg
Suggested citation for this chapter.
Harding,DP. (2022) Synthetic fertilizers: primer and raising yields. In Farmpedia, The Encyclopedia for Small Scale Farmers. Editor, M.N. Raizada, University of Guelph, Canada. http://www.farmpedia.org
Introduction
Synthetic fertilizers are concentrated, typically commercial sources of plant nutrients that are generally more stable and easier to ship than organic nutrient sources. Though the term “synthetic” is used, fertilizers are composed of naturally occurring minerals that may be processed to improve their availability to plants; the exception is nitrogen fertilizer which is extracted from the atmosphere through a catalytic process that consumes natural gas (Standage, 2009) (p. 212). Nutrients are classified broadly by the relative quantities in which they are needed for plant growth (Table 1). Macronutrient fertilizers, which are needed in relatively high amounts, include nitrogen (N), phosphorus (P), and potassium (K). The secondary nutrient fertilizers are sulphur (S), calcium (Ca), and magnesium (Mg), which are needed in moderate amounts for plant growth. Micronutrient fertilizers include zinc (Zn), molybdenum (Mo), manganese (Mn), iron (Fe), copper (Cu), and boron (B), all of which plants need in relatively small amounts (Principals of Plant Nutrition, 2001)(p2).
Each fertilizer required by plants has a unique, natural biological function. Nitrogen is a building block for amino acids (p 419), which make up proteins, while phosphorus is a component of DNA (p 467), and potassium is required to maintain hydraulic pressure within plant cells and transmit biological signals (p 492) (Principals of Plant Nutrition, 2001). Similarly, each of the secondary and micro- nutrients have specific functions within plants: for example zinc is required for approximately 100 “master genes” in plants to function properly (Li et al., 2013). Molybdenum is required for biological nitrogen fixation by bacteria that are symbiotic with legumes (Bambara & Ndakidemi, 2010).
Synthetic fertilizers can contain one or more of the 12 plant nutrients, but some governments promote formulations that contain only N or N+P, which can become less effective as other minerals are depleted from the soil. Similarly, many farmers are primarily concerned with supplying the macronutrients (N, P, and K) to the soil, and in this situation shortages of secondary and micro- nutrients can as limiting on plant growth as a macronutrient deficiency.
There are some negative opinions towards synthetic fertilizer use, some of which is justified (Rees et al., 2013). Firstly, because synthetic fertilizers are associated with industrial chemical agriculture, they are commonly equated with pesticides, despite their inherent differences. Second, production of the non-mined fertilizer, nitrogen (ammonia, nitrate, urea), consumes natural gas and hence contributes to global warming (Rees et al., 2013). Finally, the concentrated application of most synthetic fertilizers can potentially create an imbalance and lead to pollution of nearby ecosystems (Badruzzaman, Pinzon, Oppenheimer, & Jacangelo, 2012). However, when responsibly used, synthetic fertilizer can significantly improve yields and can improve the overall sustainability of agroecosystems that face absolute shortages of minerals necessary for plant growth (Principals of Plant Nutrition, 2001)(p 338).
Each nutrient has a specific function within the plant, and thus an abundance of one nutrient will not make up for a shortage of another. For this reason it is important that an appropriate balance of synthetic fertilizers and/or organic nutrient sources (e.g. manure) is employed in order to provide all of the necessary nutrients for plant growth.
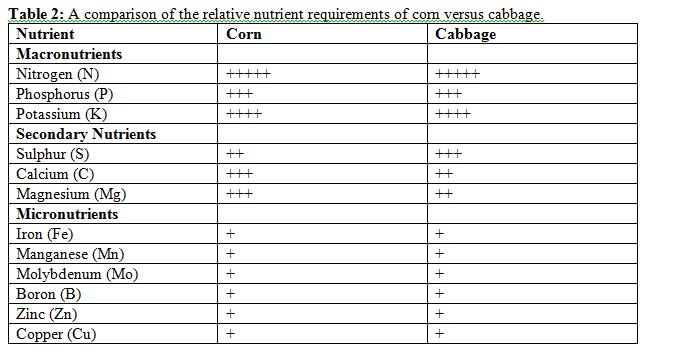
Though all 12 nutrients are required for plant growth, every crop requires these nutrients in a specific ratio. To illustrate this point, the approximate ratio of nutrients required by corn and cabbage are provided in Table 2 below. Commercial fertilizers (and organic nutrient sources) will contain different combinations and ratios of nutrients (see Fertilizer Formulation section). In some areas of the world (Ethiopia, Haiti), government policies have made primarily only one fertilizer blend available to farmers.
Along with the different materials gloves are made of, there are also different arm lengths. Some gloves are cut off just in front or around the wrist. While others can be up to and over the elbow and everywhere in between (Melco, 2016). The benefits of the shorter gloves is comfort, no bunching around wrist or elbow, and they can be quickly put on or removed. The benefits of the long gloves are more protection, the entire forearm will be covered. All the while there is less of a chance of getting debris in their gloves because the opening is farther away from what you are working with. Farmers can also work in deeper water or mud with the long rubber gloves without getting your hands wet.
Adapted from (Principals of Plant Nutrition, 2001)(p340)
The above ratios should be interpreted as a general guideline. Where available, specific crop requirement guidelines based on local trials should be used. In general, cereals such as corn, rice, millet, sorghum and wheat will require more nitrogen than any of the other nutrients (Principals of Plant Nutrition, 2001)(p369). For legumes such as soybean, common bean, cowpea, chickpea, lentils, and peanut (groundnut), phosphorus and potassium are needed from the soil in the greatest amounts (Principals of Plant Nutrition, 2001)(p369). For starchy crops such as cassava, potatoes, sweet potatoes, yams, bananas and plantains, potassium is generally the most needed nutrient (Principals of Plant Nutrition, 2001)(p369). Sulphur is also commonly limiting in tropical soils (B. K. R. Rao et al., 2012). Although this is the general trend for nutrient requirements, there is great variation according to soil type, climate, and management history, and shortages of additional minerals can also become limiting if they are not being added to the soil.
Mobile and Immobile Nutrients
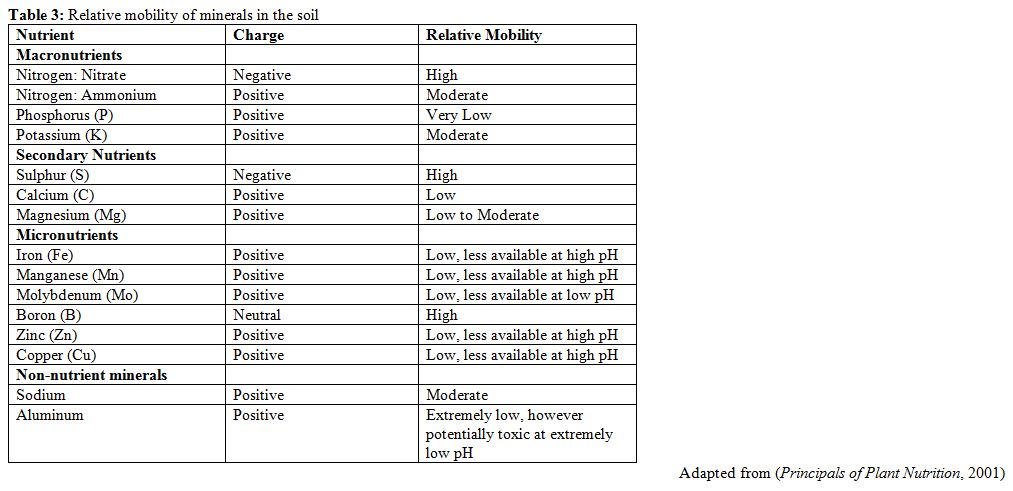
Fertilizers can be leached from the soil or lost after application, which wastes money, however this vulnerability varies between nutrients. In general, positively charged nutrients will be attracted to soil particles (which tend to be negatively charged) and are thus less prone to loss, whereas negatively charged or neutral nutrients (sulphur, nitrogen in nitrate form, and boron) travel in soil water and are easily lost from the soil (Principals of Plant Nutrition, 2001). Although the relative risk of nutrient loss is lower with positively charged nutrients, it can still occur and any synthetic fertilizer should be applied shortly before planting, ideally after heavy rains have occurred (Principals of Plant Nutrition, 2001)(p342).
Most crops require high levels of nitrogen for their growth, which can be an especially great challenge as nitrogen is also one of the most easily lost nutrients (Principals of Plant Nutrition, 2001)(p342). There is some evidence that nitrogen will be more used more efficiently in certain crops if a portion of the nitrogen application is delayed until a crop’s root system has developed (Mohammed et al., 2013). Nitrogen can also easily be converted into a gaseous form and for this reason there is some indication that incorporating synthetic nitrogen fertilizers into the soil can also increase uptake efficiency (Schnier, Dedatta, Mengel, Marqueses, & Faronilo, 1988).
Commercially produced “super-phosphate” fertilizers and their derivatives are highly plant available, however it has been reported that they can become bound to soil particles, especially in highly-weathered tropical soils, limiting their effect (van Straaten, 2002).This phenomenon has been reported to be especially severe in acidified soils (Principals of Plant Nutrition, 2001).There is some evidence that phosphorus availability can be increased by applying it in concentrated bands beneath the soil surface, as this will reduce the degree of direct contact between phosphorus and soil particles (Principals of Plant Nutrition, 2001)(p367).
Over-application of any nutrient can cause damage (often referred to as “burning”) or even death to a growing crop (Principals of Plant Nutrition, 2001). For this reason it is very important to follow nutrient application guidelines for the crops under cultivation, and to ensure that fertilizer is spread evenly throughout the field. See Practical Tips, below, for suggestions on applying fertilizer evenly. Much of the way nutrients move through the soil has to do with their individual static electrical charges (Principals of Plant Nutrition, 2001)(Ch. 2). Positively charged nutrients will naturally reach a balance between particles that are directly associated with the soil and particles that will exist in soil water (Principals of Plant Nutrition, 2001)(Ch 2.1.2). The degree of attraction between a positively charged nutrient and the soil will vary depending on both the nutrient and the soil type, however it is important to keep in mind that in most situations a certain fraction of each nutrient will exist in soil water and thus be somewhat mobile. The relative mobility of common minerals in the soil is described in Table 3.
Unlike most plant nutrients, sulphur and some forms of nitrogen (nitrate) exist in the soil as negatively charged molecules (Principals of Plant Nutrition, 2001). As negatively charged nutrients are not attracted to soil particles, they will travel exclusively in the soil water and are thus much more mobile. Similarly, boron (the only uncharged nutrient) has no attraction to soil particles and is also highly mobile (Principals of Plant Nutrition, 2001)(p621). Because of these unique properties, the behaviour of these nutrients is more affected by soil texture than those that are positively charged. In general, as sandier (often referred to as “red”) soils drain more quickly, there will be a much higher risk of leaching loss for these nutrients. There is a lower risk of loss through leaching on clay (often referred to as “brown” or “black”) soils, however waterlogged conditions (more common to clay) can also cause loss of nitrogen and sulphur through gasification (Principals of Plant Nutrition, 2001).
As noted above, splitting nitrogen application into two doses (one before planting, followed by a second application into an already established crop) has been shown to improve yield in wheat and corn (Abbasi, Tahir, & Rahim, 2013; Mohammed et al., 2013), however this trend is not consistent for all crops (Zebarth, Leclerc, & Moreau, 2004). The most reliable way to determine if splitting nitrogen application into two doses will be beneficial is through setting up a small trial plot that compares split application of nitrogen to the current practice. If split application confers a yield benefit with the local combination of crop and soil, then wider application of this practice can be considered.
Some commercial fertilizers are prepared with an outer coating that slows the release of nutrients into the soil (Notario Del Pino, Arteaga Padron, Gonzalez Martin, & Garcia Hernandez, 1995). This is valuable as an insurance against nutrient loss and also to decrease the risk of over-applying a particular nutrient, which can damage crops. Other techniques such as incorporating synthetic fertilizers into charcoal have also shown some potential to slow the release of nutrients (Khan et al., 2008), however the additional labour required by this technique may not justify the practice depending on the nutrient loss potential of the soil.
Nutrient Forms
Nitrogen
Most plant nutrients can only be taken up by plants as fairly small, simple particles. Most commercial fertilizers are composed of such particles that are immediately plant available, or will quickly break down to these forms once introduced into the soil. Organic material on the other hand must be broken down by decomposer organisms before the minerals it contains are available to plants. The slower release of minerals from organic matter decreases the risk of loss, although it should be noted that organic matter may not be an independently reliable source of nutrients. For more information on using organic matter as a nutrient source please see Chapter 3: Balanced Fertilization.
Plant available (often referred to as “mineral” nitrogen) can refer to either ammonium (NH4+) or nitrate (NO3-). There is also some evidence that small organic nitrogen containing molecules such as amino acids can be taken up directly by plants (Jones & Darrah, 1993; Warren, 2013), however almost all synthetic fertilizers will contain minerals forms of nitrogen. Both nitrate and ammonium molecules are plant available, although it has been recognized that some plant varieties have a preference for one or the other (Zhao et al., 2013). However, in most situations either source of nitrogen will be equally effective (Mengel and Kirkby, 2001 p431). Common forms of N fertilizer and notes on their individual characteristics are summarized in Table 4.
Ammonium is at lower risk of loss through leaching from the soil because its positive charge causes it to be attracted to soil particles, whereas nitrate will exist almost exclusively in the soil water and thus can easily be lost. In many soils ammonium will quickly be converted to nitrate by bacteria through a process called nitrification, thus increasing the risk of loss through leaching (Principals of Plant Nutrition, 2001)(pg 410). Additionally, in waterlogged soils where oxygen is a limiting factor, nitrate can be converted to a gaseous form by soil bacteria and lost to the atmosphere (Principals of Plant Nutrition, 2001). Ammonium will rapidly convert to ammonia gas in soils with especially high pH (loss begins around pH of 8, and increases with alkalinity). For this reason, ammonium containing fertilizers should not be applied to highly alkaline soils or mixed with calcium containing compounds (such as lime) as this can cause acute pH increase and gasification of nitrogen (Principals of Plant Nutrition, 2001)(p432).
Soil tests for levels of nitrate and ammonium levels will generally only remain accurate for a few days because mineralization of organic nitrogen and loss of nitrogen to the environment will quickly cause variation from the test levels. For this reason, soil testing of nitrogen levels should be done within a few days of fertilizer application to ensure that an appropriate fertilizer rate is calculated. As most crops will need a fairly high quantity of nitrogen (legumes are a notable exception) and because nitrogen is highly prone to loss, it is usually a safe assumption that nitrogen will be needed by crops in some degree.
Phosphorus
Phosphorus fertilizers are also available in a variety of forms. Some basic information on a few of the most common sources of phosphorus is provided in Table 5.
Phosphorus can exist in many different forms within the soil, of which each has varying degrees of availability to plants(van Straaten, 2002). There are many deposits of phosphate-containing rock throughout the world which could potentially be employed as crop amendments, however there is wide variation in the composition of the rock and its suitability for agriculture (van Straaten, 2002). For more information on phosphate rock and other useful mineral sources for farms, please see “Rocks for Crops: Agrominerals of sub-Saharan Africa” by Peter van Straaten (2002). A link to an online version of this book is provided at the end of this chapter.
Understanding Fertilizer Formulations
Most commercial fertilizer formulations will be defined by an NPK value that will appear as three numbers separated by dashes such as 10-10-10 or 46-0-0. These numbers represent the percent concentration of nitrogen, phosphorus and potassium, respectively, in the fertilizers.
Nitrogen is expressed in fertilizer formulations as the percent of the total weight of fertilizer that is elemental nitrogen (i.e. made up of nitrogen atoms), whereas phosphorus and potassium are expressed as percent of the total weight that is phosphorus or potassium in an oxidized form (P2O5 and K2O, respectively). This is important to note because the oxidized form of a mineral will be heavier due to the attached oxygen molecules. For example, a portion of fertilizer that is 16% oxidized phosphorus will contain less actual phosphorus than an equal portion of fertilizer that is 16% elemental phosphorus (weight made up exclusively by phosphorus atoms).
Most fertilizer recommendations will be expressed in terms of elemental nitrogen and oxidized phosphorus and potassium, so conversion of units is not necessary. However, one must be careful when interpreting soil test results to ensure that a suggested fertilization rate for P or K in an elemental form is not mistaken for a fertilization rate for P or K in an oxidized form, or visa versa. Conversation factors for elemental P to P2O5 and elemental K to K2O are provided below:
P = P2O5 / 2.29 1 gram of elemental phosphorus would weight 2.29 g if fully oxidized
P2O5 = 2.29 x P 1 gram of P2O5 contains 0.44 g of elemental phosphorus
K = K2O /1.21 1 gram of elemental potassium would weigh 1.21 g if fully oxidized
K2O = 1.21 x K 1 gram of K2O contains 0.83 grams of elemental potassium
Adapted from Colorado State University Extension Fact Sheet no. 0.548 available online at: http://www.ext.colostate.edu/pubs/crops/00548.pdf
The ideal mixture and total quantity of fertilizers to be applied should be based on the approximate quantity of each nutrient already in the soil, the relative requirement of the crop for each nutrient, and the nutrient holding capacity of the soil. Additionally, the cost of fertilizer in relation to its effect on yield should be considered, as the quantity that maximizes yield may be prohibitively expensive or simply greater than the quantity that will maximize profit. In situations where fertilizer is highly expensive, a low rate may be the most effective in generating a profitable yield.
Fertilizer Manufacturing
Most fertilizers are derived from mineral deposits within the earth that are accessed through mining. In some cases minerals will be suitable for application immediately upon extraction however further processing is often employed to ensure the purity and plant availability of the final product. For example, phosphorus is generally mined as rock phosphate, which is composed of tightly bound phosphorus and calcium (van Straaten, 2002). As rock phosphate only minimally available to plants, it is commonly treated with sulfuric acid to convert it to H2PO4 or “Super-phosphate” (Principals of Plant Nutrition, 2001)(p 473).
Unlike other nutrients, synthetic nitrogen produced through a chemical reaction involving air and hydrogen gas. Our air is about 80% nitrogen, in the form of highly stable N2 gas. This gas can however be converted into ammonia (NH3) through natural enzymatic reactions (such as those performed by bacteria that live inside legumes) or through an industrial method known as the Haber-Bosch Process (Principals of Plant Nutrition, 2001). Through combining the N¬2 gas in the air with hydrogen gas under extremely high temperature and pressure in the presence of an appropriate catalyst, ammonia will be produced. Ammonia is a highly reactive gas which can be applied directly to soil using specialized equipment however it is usually processed further into more stable forms such as urea (Principals of Plant Nutrition, 2001). The hydrogen gas used in this reaction is generally derived from natural gas, and for this reason the market price of ammonia based fertilizer will generally follow the market price of natural gas.
Practical Considerations
Fertilizers cost money and unfortunately are required at a point in the growing season when many farmers have little available cash. Small, short-term loans and other micro-financing arrangements are often very effective tools to improve agricultural productivity by enabling farmers to afford fertilizer before profits from harvest have been realized.
Unfortunately there have been cases of fraudulent fertilizer sales where the product being sold either did not contain the advertised nutrients or was highly diluted. For this reason it is important to purchase fertilizers from credible dealers. When uncertain of product quality but faced with few other options, performing a small split plot trial (described below) can be an effective method of assessing fertilizer value before investing heavily.
It should be noted that the availability and behaviour of many crop nutrients is highly influenced by soil acidity. For more information on this relationship please see Chapter 8: Soil Acidity.
The highly concentrated nature of synthetic fertilizers increases the risk of problems stemming from applying too much at once. As noted above, too much of any nutrient can damage a plant, symptoms that are commonly referred to as “burning” (Principals of Plant Nutrition, 2001). Additionally, the entry of plant nutrients into groundwater through leaching can cause environmental imbalance as well as human health risks associated with contaminated well water. Loss of nutrients through surface runoff can also cause imbalance to local ecosystems, particularly when field runoff enters waterways (Badruzzaman et al., 2012). The economic consequences of these losses can be very significant for many small share farmers.
Fertilizer can be applied to a field through a variety of methods. Broadcasting, or spreading fertilizer evenly over the soil surface is the most common method of fertilizer application. This is often followed by working the fertilizer into the soil through tillage to reduce the risk of nutrient loss and to increase the proximity of the fertilizer to plant roots for less mobile nutrients. Fertilizer can also be placed in shallow trenches between crop rows that can be made with a furrow and then covered, a process referred to as side-banding. Microdosing is another method of fertilizer application that is most appropriate for situations where applying high rates of fertilizer is impractical or prohibitively expensive.
When broadcasting fertilizer it is important to ensure it is spread evenly in order to avoid concentrated pockets of fertilizer that can cause crop burning and nutrient loss. Mechanical broadcast spreaders should be tested before use to ensure they are functioning properly. When spreading fertilizer by hand, it can be helpful to divide the field into small subsections with posts and string. Using a container marked to indicate the proper volume of fertilizer per section, sections can be fertilized one by one ensuring that the appropriate amount of fertilizer is spread evenly within each subsection.
Coarse soils, especially in tropical areas generally have lower nutrient holding capacities and are especially prone to leaching loss (Principals of Plant Nutrition, 2001). For this reason it is important to avoid over-application of fertilizer as nutrients that are not held in the soil can easily be lost to leaching. Fertilizer recommendations for North America and Europe are generally intended for soils with higher nutrient holding capacities than what tropical soils can carry (van Straaten, 2002). Thus, it is important to use locally generated fertilizer recommendations where available and to err of the side of caution when applying fertilizer.
As most nutrients are taken up by plants in soil water, it is important that there is some degree of moisture in the soil when fertilizer is applied or shortly thereafter. Although it cannot be stressed enough that heavy rainfall can cause significant or total fertilizer loss, a moderate degree of soil moisture is necessary to ensure that the nutrients in the fertilizer can make their way to plants.
Commercial fertilizers are most commonly available in powdered or granulated forms, however liquid formulations are also available. Liquid formulations may be more convenient to apply in some situations however they are also generally more expensive. There is no inherent advantage to using a liquid fertilizer apart from the possibility of more convenient application depending on management style.
To fully ensure that a fertilizer will be effectively both economically and in terms of yield response, testing a given fertilizer combination in a small trial plot before widespread application is often a wise precaution, especially when fertilizer is a significant expense.
Picture Based Lesson to Train Farmers
For the South Asian version (pictures only, text for you to insert), click this link for lesson 5.16:http://www.sakbooks.com/uploads/8/1/5/7/81574912/5.16_south_asian.pdf
For the East/South Asian version (pictures only, text for you to insert), click this link for lesson 5.16:http://www.sakbooks.com/uploads/8/1/5/7/81574912/5.16e.s.a.pdf
For the Sub-Saharan Africa/Caribbean version (pictures only, text for you to insert), click this link for lesson 5.16:http://www.sakbooks.com/uploads/8/1/5/7/81574912/5.16subsaharan_africa_carribean.pdf
For the Latin-America version (pictures only, text for you to insert), click this link for lesson 5.16:http://www.sakbooks.com/uploads/8/1/5/7/81574912/5.13latin_america.pdf
For North Africa And Middle East version (pictures only, text for you to insert), click this link for lesson Chapter 5. 4.12:http://www.sakbooks.com/uploads/8/1/5/7/81574912/4.12n._africa_middleeast.pdf
Source: MN Raizada and L Smith (2016) A Picture Book of Best Practices for Subsistence Farmers. eBook, University of Guelph Sustainable Agriculture Kit (SAK) Project, June 2016, Guelph, Canada.
Practical Links
The International Plant Nutrition Institute (IPNI) offers several up to date publications on best management practices for fertilizer use:
[Another guide to the basics of fertilizer use is available from VirginiaTech: http://www.dcr.virginia.gov/stormwater_management/documents/nmagscsthe01.pdf]
References
1.Abbasi, M. K., Tahir, M. M., & Rahim, N. (2013). Effect of N fertilizer source and timing on yield and N use efficiency of rainfed maize (Zea mays L.) in Kashmir-Pakistan. Geoderma, 195, 87-93.
2.Badruzzaman, M., Pinzon, J., Oppenheimer, J., & Jacangelo, J. G. (2012). Sources of nutrients impacting surface waters in Florida: A review. Journal of Environmental Management, 109, 80-92. doi: 10.1016/j.jenvman.2012.04.040.
3.Bambara, Sylvie, & Ndakidemi, Patrick A. (2010). Phaseolus vulgaris response to Rhizobium inoculation, lime and molybdenum in selected low pH soil in Western Cape, South Africa. African Journal of Agricultural Research, 5(14), 1804-1811.
4.Jones, D. L., & Darrah, P. R. (1993). INFLUX AND EFFLUX OF AMINO-ACIDS FROM ZEA-MAYS L ROOTS AND THEIR IMPLICATIONS FOR N-NUTRITION AND THE RHIZOSPHERE. Plant and Soil, 155, 87-90. doi: 10.1007/bf00024990.
5.Khan, Modabber Ahmed, Kim, Ki-wook, Mingzhi, Wang, Lim, Bu-kug, Lee, Weon-hee, & Lee, Jong-yoon. (2008). Nutrient-impregnated charcoal: an environmentally friendly slow-release fertilizer. The Environmentalist., 28(3), 231-235.
6.Li, S. Y., Zhao, B. R., Yuan, D. Y., Duan, M. J., Qian, Q., Tang, L., . . . Li, C. Y. (2013). Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression. Proceedings of the National Academy of Sciences of the United States of America, 110(8), 3167-3172. doi: 10.1073/pnas.1300359110.
7.Mohammed, Y. A., Kelly, J., Chim, B. K., Rutto, E., Waldschmidt, K., Mullock, J., . . . Raun, W. (2013). NITROGEN FERTILIZER MANAGEMENT FOR IMPROVED GRAIN QUALITY AND YIELD IN WINTER WHEAT IN OKLAHOMA. Journal of Plant Nutrition, 36(5), 749-761. doi: 10.1080/01904167.2012.754039
8.Notario Del Pino, J. S., Arteaga Padron, I. J., Gonzalez Martin, M. M., & Garcia Hernandez, J. E. (1995). Phosphorus and potassium release from phillipsite-based slow-release fertilizers. Journal of Controlled Release, 34(1), 25-29. doi: 10.1016/0168-3659(94)00116-c.
9.Rao, B. K. Rajashekhara, Krishnappa, K., Srinivasarao, C., Wani, S. P., Sahrawat, K. L., & Pardhasaradhi, G. (2012). Alleviation of Multinutrient Deficiency for Productivity Enhancement of Rain-Fed Soybean and Finger Millet in the Semi-arid Region of India. Communications in Soil Science and Plant Analysis, 43(10), 1427-1435. doi: 10.1080/00103624.2012.670344.
10.Rees, R. M., Baddeley, J. A., Bhogal, A., Ball, B. C., Chadwick, D. R., Macleod, M., . . . Williams, J. R. (2013). Nitrous oxide mitigation in UK agriculture. Soil Science and Plant Nutrition, 59(1), 3-15. doi: 10.1080/00380768.2012.733869.
11.Schnier, H. F., Dedatta, S. K., Mengel, K., Marqueses, E. P., & Faronilo, J. E. (1988). NITROGEN USE EFFICIENCY, FLOODWATER PROPERTIES, AND N-15 BALANCE IN TRANSPLANTED LOWLAND RICE AS AFFECTED BY LIQUID UREA BAND PLACEMENT. Fertilizer Research, 16(3), 241-255. doi: 10.1007/bf01051374.
12.Warren, Charles R. (2013). Quaternary ammonium compounds can be abundant in some soils and are taken up as intact molecules by plants. The New phytologist, 198(2), 476-485. doi: 10.1111/nph.12171.
13.Zebarth, B. J., Leclerc, Y., & Moreau, G. (2004). Rate and timing of nitrogen fertilization of Russet Burbank potato: Nitrogen use efficiency. Canadian Journal of Plant Science, 84(3), 845-854.
14.Zhao, X. Q., Guo, S. W., Shinmachi, F., Sunairi, M., Noguchi, A., Hasegawa, I., & Shen, R. F. (2013). Aluminium tolerance in rice is antagonistic with nitrate preference and synergistic with ammonium preference. Annals of Botany, 111(1), 69-77. doi: 10.1093/aob/mcs234.